Efecto del precursor de calcio en las propiedades estructurales y microestructurales de la hidroxiapatita
Calcium precursor effect on structural and microstructural properties of hydroxyapatite
Barra lateral del artículo
Términos de la licencia (VER)

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Declaración del copyright
Los autores ceden en exclusiva a la Universidad EIA, con facultad de cesión a terceros, todos los derechos de explotación que deriven de los trabajos que sean aceptados para su publicación en la Revista EIA, así como en cualquier producto derivados de la misma y, en particular, los de reproducción, distribución, comunicación pública (incluida la puesta a disposición interactiva) y transformación (incluidas la adaptación, la modificación y, en su caso, la traducción), para todas las modalidades de explotación (a título enunciativo y no limitativo: en formato papel, electrónico, on-line, soporte informático o audiovisual, así como en cualquier otro formato, incluso con finalidad promocional o publicitaria y/o para la realización de productos derivados), para un ámbito territorial mundial y para toda la duración legal de los derechos prevista en el vigente texto difundido de la Ley de Propiedad Intelectual. Esta cesión la realizarán los autores sin derecho a ningún tipo de remuneración o indemnización.
La autorización conferida a la Revista EIA estará vigente a partir de la fecha en que se incluye en el volumen y número respectivo en el Sistema Open Journal Systems de la Revista EIA, así como en las diferentes bases e índices de datos en que se encuentra indexada la publicación.
Todos los contenidos de la Revista EIA, están publicados bajo la Licencia Creative Commons Atribución-NoComercial-NoDerivativa 4.0 Internacional
Licencia
![]()
Esta obra está bajo una Licencia Creative Commons Atribución-NoComercial-NoDerivativa 4.0 Internacional
Contenido principal del artículo
Resumen
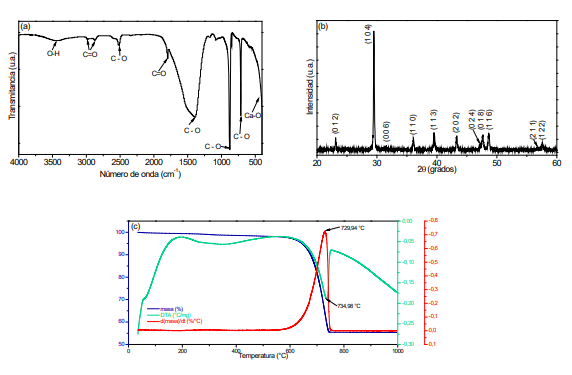
Debido a que en la actualidad las afecciones óseas siguen siendo un desafío clínico significativo y las soluciones son limitadas y en algunos casos poco efectivas, la investigación alrededor de la hidroxiapatita, principal componente mineral del hueso ha cobrado una importancia relevante. En este trabajo de investigación se analizó el efecto del precursor de calcio en las características estructurales y microestructurales de la hidroxiapatita, comparando los resultados obtenidos con hidroxiapatita extraída de una fuente natural. Mediante el método de reacción por combustión en solución fueron sintetizados polvos de hidroxiapatita utilizando como precursores de calcio carbonato de calcio extraído de la cáscara de huevo y carbonato y nitrato de calcio comerciales. A su vez, la fuente natural de hidroxiapatita fue hueso bovino, que se sometió a un proceso de lavado, fractura y tratamiento térmico. Los grupos funcionales presentes en las muestras obtenidas fueron determinados mediante espectroscopia infrarroja y las fases cristalinas mediante difracción de rayos-X. La microscopía electrónica de transmisión permitió determinar la morfología esférica de las partículas obtenidas a partir de carbonato de calcio (de cáscara de huevo) con el menor tamaño de partícula (entre 20 y 50 nm); mientras que, las obtenidas a partir de precursores comerciales presentaron una morfología no homogénea. Los resultados mostraron que el proceso seguido fue eficiente para la obtención de nanopartículas de hidroxiapatita cuando se obtiene a partir de carbonato de calcio y a una temperatura de 1100ºC. El carbonato de calcio proveniente de la cáscara de huevo permitió obtener hidroxiapatita con morfología homogénea y tamaño nanométrico.
Descargas
Detalles del artículo
Referencias (VER)
Abere, D.V.; Ojo, S.A.; Oyatogun, G.M.; Paredes-Epinosa, M.B.; Dharsika Niluxsshun, M.C.; Hakami, A. (2022). Mechanical and morphological characterization of nano-hydroxyapatite (nHA) for bone regeneration: A mini review. Biomedical Engineering Advances, 4, 100056. https://doi.org/10.1016/j.bea.2022.100056
Bahloul, L.; Azzi, A.; Maradi, H. (2020): Study of The Porosity and Density of Synthetically Produced Hydroxyapatite. SAJ Biotechnology, 7, 1, pp. 1-5.
Bantikatla, H.; N.S.M.P., Latha Devi; Bhogoju, R.K. (2021). Microstructural parameters from X-ray peak profile analysis by Williamson-Hall models; A review. Materials Today: Proceedings, 47(14), pp. 4891-4896. https://doi.org/10.1016/j.matpr.2021.06.256
Barrett, E.P.; Brown, J.M.; Oleck, S.M. (1951). Some granular carbonaceous adsorbents for sugar refining. Industrial & Engineering Chemistry Research., 43(3), pp. 639-654. https://doi.org/10.1021/ie50495a026
Chen, L.J.; Chen, T.; Cao, J.; Liu B.L.; Shao, C.S; Zhou, K.C.; Zhang, D. (2018). Effect of Tb/Mg doping on composition and physical properties of hydroxyapatite nanoparticles for gene vector application. Transactions of Nonferrous Metals Society of China, 28(1), pp. 125-136. https://doi.org/10.1016/S1003-6326(18)64645-X
De Carvalho, B.; Rompen, E.; Lecloux, G.; Schupbach, P.; Dory, E.; Art, J. F.; Lambert, F. (2019). Effect of Sintering on In Vivo Biological Performance of Chemically Deproteinized Bovine Hydroxyapatite. Materials (Basel), 12(23), 3946. https://doi.org/10.3390/ma12233946
De Witte, T.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. (2018). Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regenerative Biomaterials, 5(4), pp. 197–211. https://doi.org/10.1093/rb/rby013
Desai, K.R.; Alone, S.T.; Wadgane, S.R.; Shirsath, S.E.; Batoo, K.M.; Imran, A.; Raslan, E.H.; Hadi, M.; Ijaz, M.F.; Kadam, R.H. (2021). X-ray diffraction-based Williamson–Hall analysis and rietveld refinement for strain mechanism in Mg–Mn co-substituted CdFe2O4 nanoparticles. Physica B: Condensed Matter, 614, 413054. https://doi.org/10.1016/j.physb.2021.413054
Diningsih, C.; Rohmawati, L. (2022). Synthesis of Calcium Carbonate (CaCO3) from Eggshell by Calcination Method. Indonesian Physical Review, 5(3), pp. 208-215.
Dorozhkin, S.V. (2013). A detailed history of calcium orthophosphates from 1770s till 1950. Materials Science and Engineering: C, 33(6), pp. 3085-3110. https://doi.org/10.1016/j.msec.2013.04.002
Ebrahimi, P.; Kumar, A.; Khraisheh, M. (2022). Analysis of combustion synthesis method for Cu/CeO2 synthesis by integrating thermodynamics and design of experiments approach. Results in Engineering, 15, 100574. https://doi.org/10.1016/j.rineng.2022.100574
Fiume, E.; Magnaterra, G.; Rahdar, A.; Verné, E.; Baino, F. (2021). Hydroxyapatite for Biomedical Applications: A Short Overview. Ceramics, 4, pp. 542-563. https://doi.org/10.3390/ceramics4040039
Frikha, K.; Limousy, L.; Bouaziz, J.; Bennici, S.; Chaari, K.; Jeguirim, M. (2019). Elaboration of alumina-based materials by solution combustion synthesis: A review. Comptes Rendus Chimie, 22(2-3), pp. 206-219. https://doi.org/10.1016/j.crci.2018.10.004
Gandou, Z.; Nounah, A.; Belhorma, B.; Yahyaoui, A. (2015). Nanosized Calcium-Deficient Carbonated Hydroxyapatite synthesized by microwave activation. Journal of Materials and Environmental Science, 6(4), pp. 983-988
Hu, C.; Ashok, D.; Nisbet, D. R.; Gautam, V. (2019). Bioinspired surface modification of orthopedic implants for bone tissue engineering. Biomaterials, 219, 119366. https://doi.org/10.1016/j.biomaterials.2019.119366
Hussin, M.S.; Abdullah, H.Z.; Idris, M.I.; Wahap, M.A. (2022). Extraction of natural hydroxyapatite for biomedical applications—A review. Heliyon, 8(8), e10356. https://doi.org/10.1016/j.heliyon.2022.e10356
Irfan, H.; Racik, K; Anand, S. (2018). Microstructural evaluation of CoAl2O4 nanoparticles by Williamson–Hall and size–strain plot methods. Journal of Asian Ceramic Societies, 6(1), pp. 54-62. https://doi.org/10.1080/21870764.2018.1439606
Jain, A.; Somvanshi, A.; Prashant; Ahmad, N. (2023).X-ray diffraction analysis of SrTiO3 nanoparticles by Williamson-Hall, size-strain plot and FullProf method. Materials Today: Proceedings, in press. https://doi.org/10.1016/j.matpr.2023.03.166
Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. (2019). Bioactive calcium phosphate materials and applications in bone regeneration. Biomaterials research, 23, 4. https://doi.org/10.1186/s40824-018-0149-3
Kalita, S.J.; Bhatt, H.A. (2007). Nanocrystalline hydroxyapatite doped with magnesium and zinc, Synthesis and characterization. Materials Science and Engineering, 27(4), pp. 837-848. https://doi.org/10.1016/j.msec.2006.09.036
Kalpana, M.; Nagalakshmi, R. (2023). Effect of reaction temperature and pH on structural and morphological properties of hydroxyapatite from precipitation method, Journal of the Indian Chemical Society, 100, 100947. https://doi.org/10.1016/j.jics.2023.100947
Kubasiewicz-Ross, P.; Hadzik, J.; Seeliger, J.; Kozak, K.; Jurczyszyn, K.; Gerber, H.; Dominiak, M.; Kunert-Keil, C. (2017). New nano-hydroxyapatite in bone defect regeneration: A histological study in rats. Annals of Anatomy - Anatomischer Anzeiger, 213, pp. 83-90. https://doi.org/10.1016/j.aanat.2017.05.010
Le, B.Q.; Nurcombe, V.; Cool, S.M.; van Blitterswijk, C.A.; de Boer, J.; LaPointe, V.L.S. (2017). The Components of Bone and What They Can Teach Us about Regeneration. Materials (Basel), 11(1), 14. https://doi.org/10.3390/ma11010014
Meejoo, S.; Maneeprakorn, W.; Winotai, P. (2006). Phase and thermal stability of nanocrystalline hydroxyapatite prepared via microwave heating. Thermochimica Acta 447(1), pp. 115-120. https://doi.org/10.1016/j.tca.2006.04.013
Mohd Pu'ad, N.A.S.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. (2019). Syntheses of hydroxyapatite from natural sources. Heliyon, 5(5), e01588. https://doi.org/10.1016/j.heliyon.2019.e01588
Mohd Pu'ad, N.A.S.; Abdul Haq, R.H.; Mohd Noh, H.; Abdullah, H.Z., Idris, M.I.; Lee, T.C. (2020) Synthesis method of hydroxyapatite: A review. Materials Today: Proceedings, 29(1), pp. 233-239. https://doi.org/10.1016/j.matpr.2020.05.536
Nunes, J.P.; Neme, N.P.; de Souza Matos, M.J.; Junio, R.; Batista, C.; de Almeida Macedo, W.A.; Gastelois, P.L.; Gomes, D.A.; Rodrigues, M.A.; Cipreste, M.F.; Barros Sousa, E.M. (2023). Nanostructured system based on hydroxyapatite and curcumin: a promising candidate for osteosarcoma therapy. Ceramics International, In Press, Journal Pre-proof, https://doi.org/10.1016/j.ceramint.2023.03.115
Omori, Y.; Okada, M.; Takeda, S.; Matsumoto, N. (2014). Fabrication of dispersible calcium phosphate nanocrystals via a modified Pechini method under non-stoichiometric conditions. Materials Science and Engineering, 42, pp. 562-568. https://doi.org/10.1016/j.msec.2014.05.071
Peña, J. (2003). Hydroxyapatite, tricalcium phosphate and biphasic materials prepared by a liquid mix technique. Journal of the European Ceramic Society, 23(10), pp. 1687-1696. https://doi.org/10.1016/S0955-2219(02)00369-2
Puspitasari, P.; Utomo, D.M.; Zhorifah, H.N.; Permanasari, A.A.; Gaya, R.W. (2020). Physicochemical Determination of Calcium Carbonate (CaCO3) from Chicken Eggshell. Key Engineering Materials, 840, pp. 478-483. https://doi.org/10.4028/www.scientific.net/KEM.840.478
Qiao, D.; Cheng, S.; Xing, Z.; Zhang, Q.; Song, S.; Yan, F.; Zhang, Y. (2023). Bio-inspired glycosylated nano-hydroxyapatites enhance endogenous bone regeneration by modulating macrophage M2 polarization. Acta Biomaterialia, 162, pp. 135-148. https://doi.org/10.1016/j.actbio.2023.03.027
Rh Owen, G.; Dard, M.; Larjava, H. (2018). Hydoxyapatite/beta-tricalcium phosphate biphasic ceramics as regenerative material for the repair of complex bone defects. Journal of biomedical materials research. Part B, Applied biomaterials, 106(6), pp. 2493–2512. https://doi.org/10.1002/jbm.b.34049
Sadat-Shojai, M.; Khorasani, M.T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. (2013). Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta biomaterialia, 9(8), pp. 7591–7621. https://doi.org/10.1016/j.actbio.2013.04.012
Saxena, V.; Pandey, L.M. (2022). Synthesis and Sintering of Calcium Hydroxyapatite for Biomedical Applications, Editor(s): M.S.J. Hashmi, Encyclopedia of Materials: Plastics and Polymers, Elsevier, 859-870. https://doi.org/10.1016/B978-0-12-820352-1.00136-X
Venkateswarlu, K.; Sandhyarani, M.; Nellaippan, T.A.; Rameshbabu, N. (2014). Estimation of Crystallite Size, Lattice Strain and Dislocation Density of Nanocrystalline Carbonate Substituted Hydroxyapatite by X-ray Peak Variance Analysis. Procedia Materials Science, 5, pp. 212-221. https://doi.org/10.1016/j.mspro.2014.07.260
Zhan, J.; Tseng, Y.H.; Chan, J.C.C.; Mou, C.Y. (2005). Biomimetic formation of hydroxyapatite nanorods by a single-crystal-to-single-crystal transformation. Advanced Functional Materials, 15, pp. 2005–2010. https://doi.org/10.1002/adfm.200500274
Artículos similares
- Diego Alexander García, Diana Ali García Capdevilla, Kevin Stiven Granados Tavera, Propuesta Metodológica para Estrategias de Turismo de Aventura y de Regeneración Natural con Perspectiva Ambiental y Colaborativa en Florencia – Caquetá , Revista EIA: Vol. 22 Núm. 44 (2025): Tabla de contenido Revista EIA No. 44
También puede {advancedSearchLink} para este artículo.

 PDF
PDF
 FLIP
FLIP